Artificial Intelligence at RSNA 17: Some Observations
Posted on January 5, 2018 by Larry Sieb
 Artificial Intelligence (AI) continued to be a hot topic at the Radiological Society of North America 2017 annual meeting. It was reported that RSNA 2017 had four times the number of AI sessions as there were at RSNA 2016. Two sessions that I attended were standing room only with attendees turned away prior to the start of the papers.
Artificial Intelligence (AI) continued to be a hot topic at the Radiological Society of North America 2017 annual meeting. It was reported that RSNA 2017 had four times the number of AI sessions as there were at RSNA 2016. Two sessions that I attended were standing room only with attendees turned away prior to the start of the papers.
One of the new introductions at this meeting was the AI imaging distribution platform .
EnvoyAI (a TeraRecon company) announced its “EnvoyAI Exchange” where end users can buy access to FDA 510 (k) cleared AI algorithms and where developers can test and refine their products. At RSNA 2017, 3 algorithms on the Exchange were FDA 510(k) cleared and available for purchase. A total of 35 algorithms from 14 developers were on the exchange in various stages of development.
Nuance had a Work In Progress demonstrating its “AI Marketplace” that integrated AI applications from multiple vendors into its PowerShare image sharing platform. The marketplace will host multiple AI applications that can be selected by the user to run on specific exams in PowerShare and have the results auto populate the PowerScribe reports. Commercial availability is yet to be determined.
Siemens is adding AI applications to its “Digital Ecosystem” platform and announced Arterys as a partner in February 2017. Arterys has received FDA 510K clearance for its web-based imaging interpretation platform, MICA that supports interactive AI imaging applications. The Arterys MICA platform currently offers an AI assistant for cardiac MR image analysis that is FDA 510K cleared and has lung and liver analysis solutions pending FDA clearance.
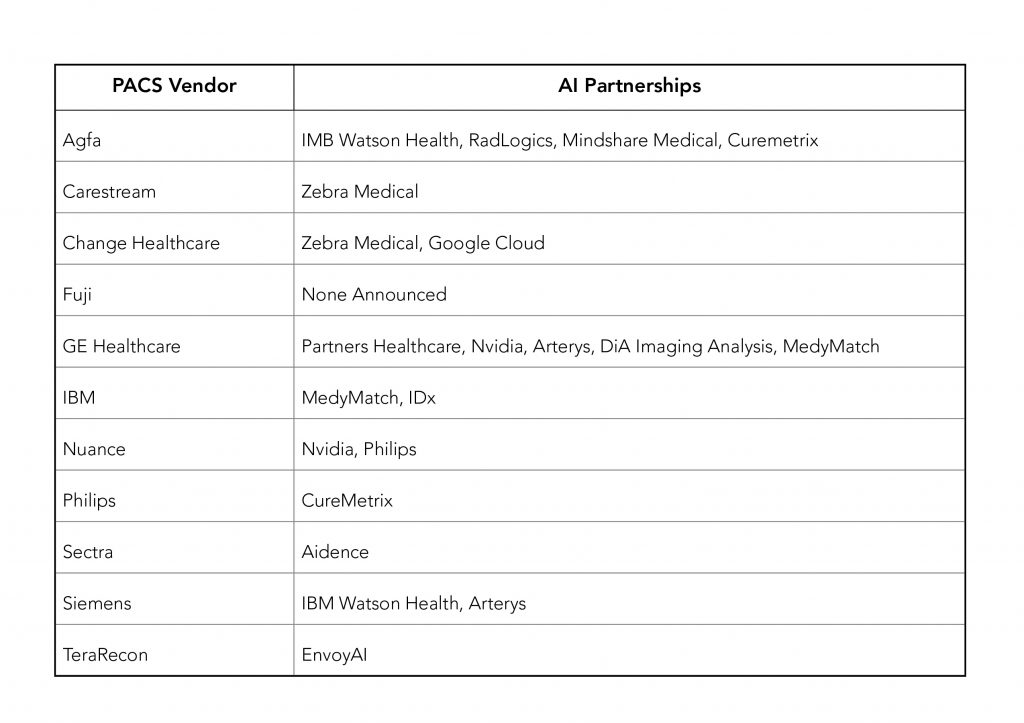
Most PACS vendors were either using 3rd parties for AI application in addition to or in lieu of, their own development. Partnerships announced as of the end of RSNA 2017 were as follows.
As of 12/28/2017, companies in the above list with FDA 510(k) clearance to market for some of their AI applications, are: Arterys, DiA Imaging Analysis(formerly DiACardio), Imbio, and RadLogics.
Presenters cautioned that deep learning neural networks are very large models that are difficult to train. Large amounts of annotated and diverse data sets are required. The models are not transparent meaning that one cannot see how the results are obtained. Validation of these models is nontrivial and will need to be done at multiple sites by clinicians. Overcoming these challenges will take time.
